Answer:
123.33mol/L
Explanations:
The formula for calculating the molarity of a solution is expressed as:
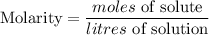
Given the following parameters
• Moles, of sucrose (solute) = 0.148 moles
• Litres ,of solution = 0.0012L
Substitute the given parameters into the formula;
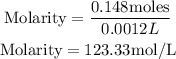
Therefore the molarity of this solution is 123.33mol/L.