We are given the liters and molarity of the solution. To find grams we must take into account the definition of molarity. Molarity is defined as:
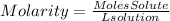
Now, we find the moles of solute:
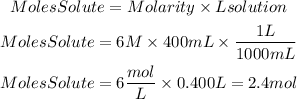
Now, to convert these moles to grams we must multiply the moles by the molar mass of Ca(NO3)2. Molar mass Ca(NO3)2 is 164.10g/mol.
So, the grams will be:
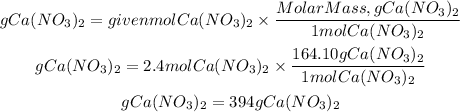
Answer: There are present 394grams of Ca(NO3)2