To determine the heat required (Q) to increase the temperature of a substance we can apply the following equation:
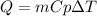
where,
m is the mass of the substance, 50g
Cp is the specific heat of the substance, 0.386J/g.K
deltaT is the temperature difference, 70K
Now, we have the values we need to calculate the heat required, we replace the known data into the equation:
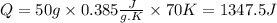
The heat required to raise the temperature of 50 g of copper by 70 K will be 1347.5J