Answer
2.12872 grams of water
Step-by-step explanation
Given:
Mass of copper (II) sulfate pentahydrate sample = 5.9 grams
What to find:
The grams of water that would be released if all of the water is removed from the 5.9 grams sample of copper (II) sulfate pentahydrate.
Step-by-step solution:
The chemical formula of copper (II) sulfate pentahydrate is: CuSO₄.5H₂O
Molar mass of CuSO₄.5H₂O = 249.68 g/mol
Mass of water in 1 mole of CuSO₄.5H₂O = 5 x molar mass of water = 5 x 18.01528 g/mol = 90.0764 g/mol
the percentage by mass of water in 1 mole of CuSO₄.5H₂O is
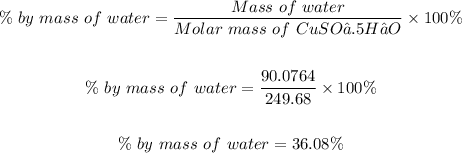
Hence, you can now use the % by mass of water in 1 mole of copper (II) sulfate pentahydrate to determine the mass of water that would be released in 5.9 grams of copper (II) sulfate pentahydrate as shown below.
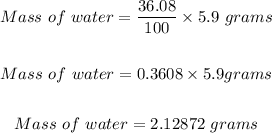
Therefore, the grams of water that would be released if all of the water is removed from the 5.9 grams of copper (II) sulfate pentahydrate is 2.12872 grams