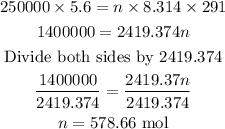Answer
578.66 mol
Step-by-step explanation
Given:
Volume, V = 5.6 L
Temperature, T = 18° C = 18 + 273 = 291 K
Pressure, P = 250 kPa = 250000 Pa
Using the ideal gas equation, we can solve for the number of moles:
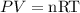
Put the given values into the ideal gas equation to get the number of moles: