The question requires us to calculate the pOH value for a solution containing 0.01 moles of HCl in 2.5 L of solution.
To solve this problem, we'll need to go through the following steps:
I) calculate the molar concentration of the HCl solution to obtaine the concentration of H+ ions;
II) using the concentration of H+ ions, calculate the pH of the solution;
III) with the value obtained for pH, calculate the value of pOH for this solution.
Next, we'll go through the steps to solve the question:
I) Since the question provided the number of moles of HCl and the volume of the solution, we can calculate the molar concentration using the following equation:
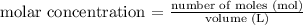
Thus, applying the values provided:
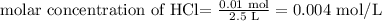
Therefore, the concentration of the HCl solution is 0.004 mol/L.
Since HCl is a strong acid, we can expect it to completely dissociate in H+ and Cl-: the concentration of H+ ions for this solution would also be 0.004 mol/L ( [H+] = 0.004 mol/L).
III) The next step is calculate the pH of this solution. We'll use the following equation and apply the concentration of H+ ions obtained in the previous step ( [) using H+] = 0.004 mol/L):
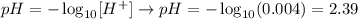
Now, we know that the pH of the solution is 2.39.
III) The last step is use the pH value calculated (pH = 2.39) to calculate the pOH of the solution, using the following equation:
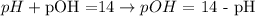
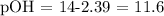
Therefore, the pOH of the HCl solution given is 11.6.