Answer:
11.7L
Explanations
According to Charles law, the volume of a given mass of gas is directly proportional to its temperature provided that the pressure is constant. Mathematically;
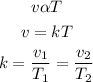
where:
• v1 and v2 are the ,initial and final ,volume
,
• T1 and T2 are the, initial and final, temperature
Given the following parameters
v1 = 8.7L
T1 = 29.0°C = 29 + 273
T1 = 302K
T2 = 133+ 273 = 406K
Substitute the given parameters into the formula
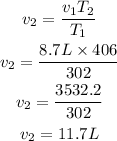
Hence the volume of the gas at 133°C is 11.7L