Answer:
142.0 moles CO₂
Step-by-step explanation:
To answer this question, we first need to know what a mole is. A mole represents
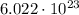 of something, and in this case, that something is molecules.
of something, and in this case, that something is molecules.
So, to convert
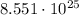 molecules into moles, we need to use the conversion factor
molecules into moles, we need to use the conversion factor
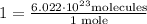 .
.
Doing so (using dimensional analysis) gives us:
