Answer
The volume of water that must be added = 2240 mL
Step-by-step explanation
Given:
Initial concentration, C₁ = 15 M
Initial volume of solution, V₁ = 560 mL
Final concentration, C₂ = 3.0 M
What to find:
The volume of water added to 560 mL of 15M H2SO4 solution to prepare a 3.0M solution.
Step-by-step solution:
The solution involves two steps.
Step 1: Calculate the final volume, V₂ of the solution using the dilution formula.
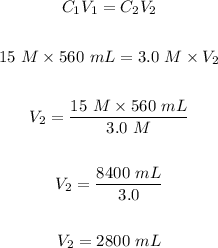
Step 2: Subtract the initial volume of the solution from the final volume in step 1 to determine the amount of water added.
The volume of water that must be added = V₂ - V₁
The volume of water that must be added = 2800 mL - 560 mL = 2240 mL