The pH of this solution is 0.207
Hello
To solve this question, we need to use the formula of solving pH of a solution from hydrogen or hydronium ion.
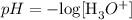
Now, we would proceed to substitute the given value and solve for it.
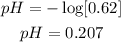
NB: we did not use the value of temperature because it is not needed in this solution.
From the calculation above, the pH of this solution is 0.207