Answer:
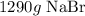
Step-by-step explanation:
Here, we want to get the number of grams in 12.5 moles NaBr
To get this, we have to multiply the number of moles by the molar mass of NaBr
The molar mass of NaBr is 103 g/mol
Thus, we have the mass as:
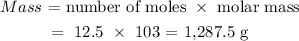
This can be approximated to 1290 which is the nearest whole number