First, let's calculate the change in temperature, which results in making the next subtraction:
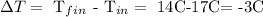
Now, let's calculate the enthalpy of the reaction with the next assumptions:
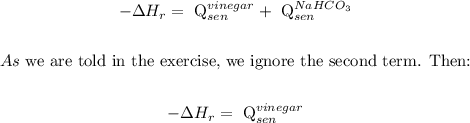
We also have to make the following assumptions: the vinegar's thermal mass and density are the same as the water's. Then, we can make the calculation:
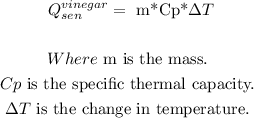
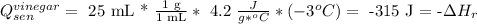
So, the reaction enthalpy equals 315 J approx.