One of the options in the question is 22.4 L, which is the value for the volume of 1mol of gas in the old STP definition. Since there is no option for the updated STP conditions, we will need to assume it want in this old one.
In the old STP conditions, 1 mol of gas occupies 22.4 L, so we can use the rule of three to calculate how many moles are in 45.0L:
22.4L --- 1mol
45.0L --- n
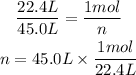
Now, we want to convert to mass. Consulting the molar mass of CO₂, we can see that it is approximately 44.01g/mol, which is one of the optios. That is, in 1 mol of CO₂ there are 44.01 g. Using the rule of three:
1 mol --- 44.01 g
n --- m
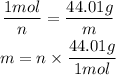
Inputting the n we calculated earlier, we have:
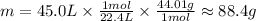
So, there is approximately 88.4g of CO₂.