Answer:
C. If the difference in electronegativity is from 0.6 to 1.7, the bond will be polar and covalent.
Step-by-step explanation:
Let's see the type of bondings based on the electronegativity differences between atoms:
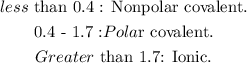
So based on this, the answer would be C. If the difference in electronegativity is from 0.6 to 1.7, the bond will be polar and covalent.