ANSWER
Ag+ is the oxidizing agent
Ag+ is reduced to Ag
Zn is oxidized to zn^2+
Zn is the reducing agent
option ABCD
Explanation:
Given information
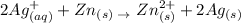
From the above redox reaction, you will see that there is a change in the oxidation number of zinc and silver.
Zn goes from 0 to 2+ in its oxidation state
Hence, zinc has been oxidized, therefore, it is a reducing agent
Ag goes from +1 to 0 in its oxidation state
Hence, Ag has been reduced, therefore, it is an oxidizing agent