In chemistry, reduction means any of a class of chemical reactions in which the number of electrons associated with an atom or a group of atoms is increased.
You can say that a compound that is gaining electrons is being reduced.
The situation where the reduction is occurring is:
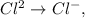
When the reduction occurs, the oxidation number (which is the superindex), it seems that is reducing this number. In this case, 2+ become 1-.
Remember: when the oxidation number is reducing, it means that the compound or element is a reduction (gaining electrons) and when the oxidation number is increasing, it means that it is oxidation (losing electrons) such as the other cases of the problem.