ANSWER
There are 3.3 moles of Cl2 in 1.1 moles of SeCl6
Step-by-step explanation
Given that
The number of moles of SeCl6 is 1.1 moles
Firstly, write a balanced equation of the reaction
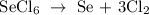
In the reaction above, 1 mole SeCl6 gives 3 moles Cl2
Let x be the number of moles of Cl2
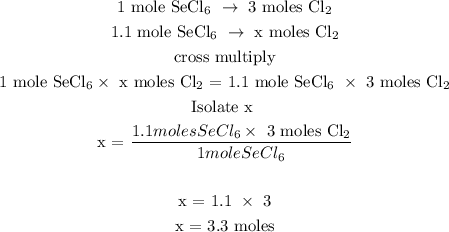
Therefore, there are 3.3 moles of Cl2 in 1.1 moles of SeCl6