To find the concentration we can do it in terms of molarity. Molarity is a way of expressing the concentration of a solute in a solution, it is expressed with the term M and can be described by the following equation:
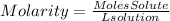
So first we must find the moles of NaCl present in 1.00 grams, the moles of NaCl will be:
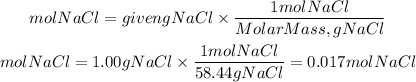
So, the molarity of the solution will be:
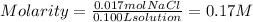
Answer: The concentration of the solution in molarity will be 0.17M