Answer: One carbon atom can make a maximum of 4 covalent bonds. The best option to answer the question is number 4.
Step-by-step explanation:
The question requires us to choose, among the options given, which one corresponds to the maximum number of carbon bonds that can be formed by one carbon atom.
To answer this question, we can consider the electronic configuration of a carbon atom. Carbon (C) presents atomic number 6, therefore it contains 6 electrons and its electronic configuration can be written as:
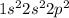
Note that there are 4 electrons in carbon's valence shell (2s2 and 2p2), thus a carbon atom needs additional 4 electrons to achieve stability.
If the atom needs 4 electrons to achieve stability, it means it can make 4 covalent bonds to "acquire" these electrons.
Therefore, the best option to answer the question is number 4.