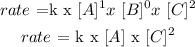Step 1
It is known that the rate law can be written as follows:
Given the reaction: X + Y => Products (completed and balanced), the rate of the reaction would be:
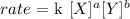
k = rate constant
[X] = concentration of reactant X
[Y] = concentration of reactant Y
a, b = are the order of the reaction with respect to X and Y (they are not necessarily the coefficients of the reaction)
---------
Step 2
Information provided:
k
First order in A
Zero order in B
Second order in C
Therefore, the rate law:
Answer: