Answer:
The specific heat of iron is 0.449 J/g°C.
Step-by-step explanation:
First, let's see the formula of specific heat:
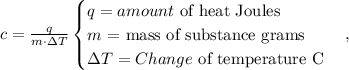
Now, we have to replace the given data in the formula, where q = 8980 J, m = 200 g and ΔT = Final temperature - Initial temperature = 120 °C - 20 °C = 100 °C:
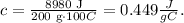
The specific heat of iron is 0.449 J/g°C.