The percent cabon in the compound is 84.22%.
1st) We need to calculate the molar mass of C4H9 with the atomic mass of carbon (C) and hydrogen (H). We can find the atomic mass in the Periodic Table of Elements:
- Carbon atomic mass: 12.01 g/mol
- Hydrogen atomic mass: 1 g/mol
The compound C4H9 has 4 carbon atoms and 9 hydrogen atoms:
(12.01 g/mol x 4) + (1 g/mol x 9) = 57.04 g/mol
So, compound C4H9 weighs 57.04g/mol.
2nd) We know that 57.04 g represents the 100% of C4H9, so with a mathematical Rule of Three we can calculate the percent carbon in C4H9 knowing that all the carbon in the molecule weighs 48.04g (12.04 g x 4):
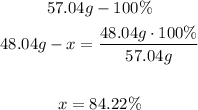
Finally, the percent cabon by mass in the compound is 84.22%.