Answer
P2 = 9.84x10^-3 kPa
Step-by-step explanation
Given:
Pressure 1 = 3.420x10^2 kPa
Volume 1 = 7.6100x10^-1 L
Temperature 1 = 1.8000x10^1 °C
Volume 2 = 1.91x10^4 L
Temperature 2 = 1.3000x10^1°C
Required: To calculate Pressure 2
Solution
We will use the combined gas law to solve this problem
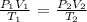
Re-arrange to make the subject P2:
P2 = P1V1T2/V2T1
P2 = (3.420x10^2 x 7.6100x10^-1 x 1.3000x10^1)/(1.91x10^4 x 1.8000x10^1)
P2 = 9.84x10^-3 kPa