Answer
979.94 grams
Step-by-step explanation
Given:
Volume of solution = 2 L
Molarity = 5 M
What to find:
The mass in grams of H3PO4 present in the solution.
Step-by-step solution:
The first step is to calculate the moles of H3PO4 present using the molarity formula below:
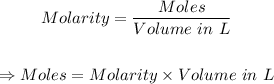
Put the values of the given parameters into the formula to get the moles:
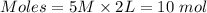
The moles of H3PO4 present in the solution = 10 mol.
Therefore, the mass present can be determined using the mole formula:
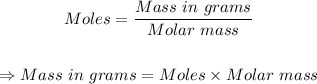
From the periodic table, the molar mass of H3PO4 can be known as 97.994 g/mol.
So putting moles = 10 mol and molar mass = 97.994 g/mol, then the mass is:
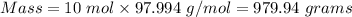
Therefore, the mass in grams of H3PO4 present in the solution = 979.94 grams.