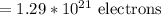
Step-by-step explanation
An Ampere is in the SI base unit of electric current equal to one coulomb per second.
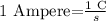
and remember that the charge of an electron is
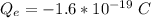
Step 1
a) let
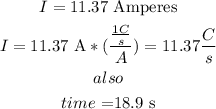
so
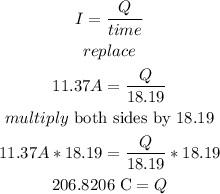
b) now , to find the number o f electrons , divide the total a}charge by the charge of an electron
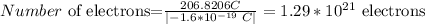
therefore, the answer is
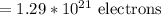
I hope this helps you