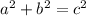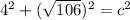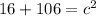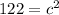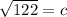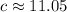Answer:
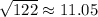
Explanation:
In any right triangle, the sum of the squares of the lengths of the legs equals the square of the length of the hypotenuse. This is commonly represented by the equation
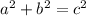 .
.
In the triangle on the left:
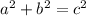
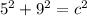
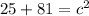
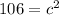
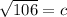
The hypotenuse of the triangle on the left becomes a leg of the triangle on the right, so we repeat the process: