Answer:
D. 16.5 moles
Step-by-step explanation:
From the balanced reaction we know that 1 mole of Zn reacts with 2 moles of HCl.
We can calculate the moles of HCl needed to react completely with 8.25 moles of zinc, using the stoichiometry of the reaction and a mathematical rule of three:
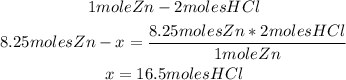
So, 16.5 moles of HCl are needed.