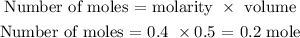Answer:
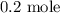
Step-by-step explanation:
Here, we want to get the number of moles of glucose in 0.5L of 0.4 M solution
Firstly, we need to recall that concentration or molarity is of the unit mol/dm^3
Mathematically, 1L is 1 dm^3 , thus, we have it that 0.4M is the same as 0.4 mol/dm^3
Now, to get the number of moles from molarity, we have to multiply the molarity by the volume
We have that as: