We have that the dilution equation of concentrated solutions will be equal to:
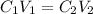
where,
C1 is the initial concentration of the solution = 7%
V1 is the initial volume = 400mL
C2 is the final concentration = 4.5%
V2 is the final volume = ?
We clear Ve and replace the known data:
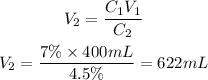
Answer: the new volume of the solution will be 622 mL