Answer:
+828kJ
Explanations:
Given the following chemical reaction with their heat of reactions
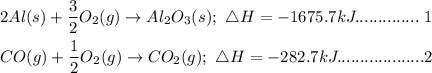
In order to arrive at the resulting reaction, we will carry out the followiing transformation;
• Swap the reactant with product, of equation 1 and;
,
• Multiply equation 2 by 3, to have;
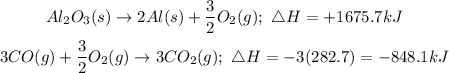
Cancel out the Oxygen element in both equation and add to have;
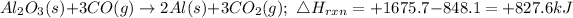
Hence the enthalpy change of the reaction to 3sf is +828kJ