Given:
• Mass of ice = 295 g ==> 0.295 kg
,
• Initial temperature, T1 = -5°C
,
• Final temperature, T2 = 20°C
Let's find the amount of heat necessary to change the ice to water.
To find the amount of heat, let's apply the Specific Heat Capacity formula:
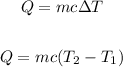
Where:
m is the mass in kg = 0.295 kg
T1 is the initial temperature
T2 is the final temperature.
c is the specific heat capacity
Let's first find the amount of heat required to change ice from -5°C to 0°C.
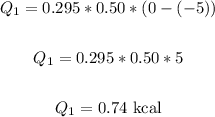
Now, let's find the heat required to change from 0°C(ice) to 0°C(water).
We have:
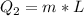
Where L is the latent fusion of water:
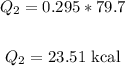
Now, let's find the heat required to change 0°C (water) to 20°C(water).
We have:
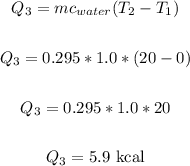
Therefore, the total heat required is:
Q = Q1 + Q2 + Q3
Thus, we have:
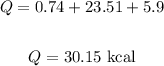
Therefore, the amount of heat neccesary is