Answer:
Explanations:
1) According to the first question, we are to find the molar mass of Na₂SO₄.
Find the molar mass of each element present on the compound
• Molar mass of Sodium (Na) = 23g/mol
,
• Molar mass of sulfur (S) = 32g/mol
,
• Molar mass of Oxygen (O) = 16g/mol
Get the molar mass of Na₂SO₄.
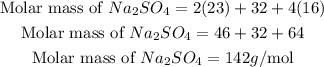
Therefore the molar mass of Na₂SO₄ is 142g/mol
2) The formula for calculating the number of moles of the compound Na₂SO₄ is expressed as:
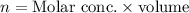
Given the following parameters:
• Molar concentration = 0.202M = 0.202mol/L
,
• Volume = 784mL= 0.784L
Substitute the given parameters into the formula:
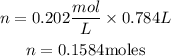
Hence the number of Na₂SO₄ needed is 0.1584moles.