Answer:
The change in enthalpy is -808 kJ.
Step-by-step explanation:
The given information from the exercise is:
C-H bonds energy: 413 kJ
O=O bonds energy: 495 kJ
C=O bonds energy: 799 kJ
H-O bonds energy: 463 kJ
Chemical reaction:
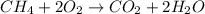
1st) It is necessary to calculate the energy of bonds broken:
4 x (C-H) = 4 x 413 kJ = 1652 kJ
2 x (O=O) = 2 x 495 kJ = 990 kJ
Total energy of bonds broken: 1652 kJ + 990 kJ = 2642 kJ
2nd) Now we have to calculate the energy of bonds made:
2 x (C=O) = 2 x 799 kJ = 1598 kJ
4 x (H-O) = 4 x 463 kJ = 1852 kJ
Total energy of bonds made: 1598 kJ + 1852 kJ = 3450 kJ
3rd) Finally, we can calculate the enthalpy for the reaction subtracting the Total energy of bonds broken minus the Total energy of bonds made:
Change in enthalpy = Total energy of bonds broken - Total energy of bonds made
Change in enthalpy = 2642 kJ - 3450 kJ
Change in enthalpy = -808 kJ
So, the change in enthalpy is -808 kJ.