Answer:
±1,±2,±3 and ±6
Step-by-step explanation:
We make use of the Rational Zero theorem below:
If a polynomial has integer coefficients, then every rational zero of f(x) has the form p/q where p is a factor of the constant term and q is a factor of the leading coefficient.
Given the function:
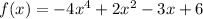
The steps to follow are given below.
Step 1: Determine all factors of the constant term and all factors of the leading coefficient.
The constant term is 6: Factors are ±1,±2,±3 and ±6
The leading coefficient is -4: Factors are ±1,±2, and ±4.
Step 2: Determine all possible values of p/q.
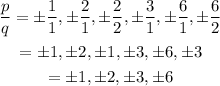
Therefore, the potential zeros are: ±1,±2,±3 and ±6.