ANSWER
Bubbles forming from hydrogen gas production
Step-by-step explanation
Given that;
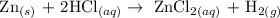
One of the method used in the preparation of hydrogen gas is the reaction of metals with acid
The above reaction is one of the method of producing hydrogen gas
When Zn reacts with HCl, bubbles formed which is as a result of hydrogen production.
Therefore, the correct answer is Bubbles forming from hydrogen gas production