The molarity of the solution is 0.209 M.
Molarity is an expression of the concentration of a compound and it is represented in mole/liter.
So, we need to know how many moles tha sample has.
- First, with the molar mass of the NaCl molecule we calculate the number of moles conteined in 9.2g of NaCl:
NaCl molar mass= 58.44g/mol
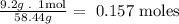
- Second, molarity is expressed in 1 liter, so we calculate the number of moles that are conteined in 1 liter:
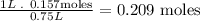
- So, the molarity of the solution is 0.209 M.