ANSWER:
250500 J
Explanation:
Given:
Mass (m) = 750 grams
We can determine the amount of heat removed by taking into account the following image:
This means that to go from water to ice there is an absorbed radiation dose of 334 J/g, therefore, the heat removed is:
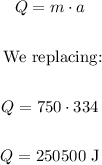
The heat removed is 250500 joules.