Answer:
The final temperature is 3,952K. (The closest option is 3,950K).
Step-by-step explanation:
The given information from the exercise is:
- Initial pressure (P1): 300.6mmHg
- Initial volume (V1): 1.23L
- Initial temperature (T1): 218.5K
- Final volume (V2): 8.32L
- Final pressure (P2): 802.75mmHg
We can calculate the final temperature (T2), by replacing the values of P1, V1, T1, V2 and P2 in the following formula of Ideal Gases:
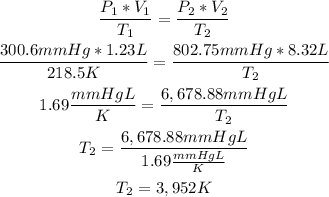
So, the final temperature is 3,952K. (The closest option is 3,950K).