Answer
Molarity = 1.28 mol/L
Step-by-step explanation
Given:
Mass of NaCl = 75 g
Volume of water = 1.0 L
What to find:
The molarity of the solution.
Step-by-step solution:
The molarity of the solution can be calculated using the molarity formula given below:
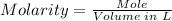
However, you need to convert the mass of NaCl given to moles using the mole formula; Mass divided by molar mass.
The molar mass of NaCl = 58.44 g/mol
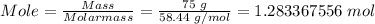
Now, the molarity is
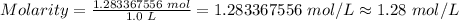
Hence, the molarity of the solution is 1.28 mol/L