ANSWER
The mass of NaOH in grams is 4.0 grams
Step-by-step explanation
Given data
The volume of the solution = 100 ml
The molarity of the solution = 1 M
To find the mass of NaOH in grams. follow the steps below
Step 1: Find the number of moles by applying the molarity formula
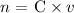
Where
n = the number of moles
C = Concentration in molarity
v = volume of the solution in liters
Step 2:convert the volume of the solution from ml to l
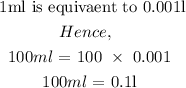
Step 3: substitute the given data into the formula in step 1
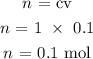
Hence, the number of moles of NaOH is 0.1 mol
Step 4: Find the mass in grams by using the below formula
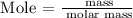
Recall, that the molar mass of NaOH is 39.997 g/mol
Step 5: substitute the given data into the formula in step 4
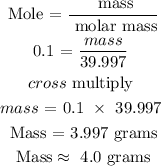
Hence, the mass of NaOH in grams is 4.0 grams