Answer:
8
Explanation:
The images that are associated with the trait 'indecisive' are 3 and 5.
• The frequency of the image 3 = 5
,
• The frequency of the image 5 = 3
Add the two:
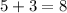
Therefore, the number of participants that selected an image that is associated with being indecisive is 8.