Answer:
n = 0.0276 mol
Step-by-step explanation:
Required: Moles of NaOH
Given:
Volume of NaOH = 21.4 mL
Molarity/concentation = 1.29 M
Solution:
We will use the following equation to solve the problem:
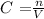
C is the concentration or molarity in mol/L or M, n is the number of moles in mol and V is the volume in L. Therefore we need to first convert mL to L.
1mL = 0.001 L
therefore 21.4 mL = 0.0214 L
Lets re-arrange the equation:
n = CV
n = 1.29 mol/L x 0.0214 L
n = 0.0276 mol