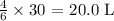Answer:
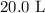
Step-by-step explanation:
Here, we want to get the volume of NH3 that will react with 30L of NO at STP
From the question, we have it that 4 moles of ammonia reacted 6 moles of NO
Thus, we have the mole ratio as 6 to 4
Thus, the volume of ammonia reacted would be: