Answer
9.987 grams of O2
Step-by-step explanation
Given:
Mass of potassium chlorate that decomposed = 25.5 g
What to find:
The mass of oxygen produced.
Solution:
The first step is to write the balanced chemical equation for the reaction.
2KClO₃ → 2KCl + 3O₂
From the equation; 2 moles of KClO₃ produced 3 moles of O₂
1 mole KClO₃ = 122.55 g/mol and 1 mole O₂ = 31.998 g/mol
This implies (2 mol x 122.55 g/mol) = 245.1 g of KClO₃ produced (3 mol x 31.998 g/mol) = 95.994 g of O₂
Therefore, 25.5 g of KClO₃ will produce
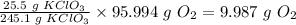
Therefore, the mass of oxygen produced is 9.987 grams