Nickel has 28 electrons, its electron configuration is:
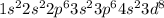
Ni2+ has lost 2 electrons, which means that the resulting configuration would be:
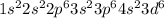
Nevertheless, since 4s electrons are further from the nucleus, they are lost resulting in a more stable ion, then, the actual configuration of Ni2+ is:
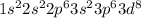
It means that this ion has 16 valence electrons.