Answer:
The density of the gas will increase.
Step-by-step explanation:
From the Charles's law of Ideal gases, we know that, if the pressure is constant, the behavior of the gas will be represented like:
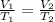
So, if the temperature of a gas is increased, the volume of the gas will decrease, because temperature and volume (in the charles's law, that is with constant pressure) are inversely proportional.
Now, to analyze what happens with the density, we can see the density formula:
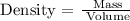
So, if the volume of the gas decrease, the density of the gas will increase, because in the density formula, volume and density are inversely proportional.
Finally, if the temperature of a gas is increased and the pressure is held constant, the density of the gas will increase.