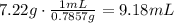We can use density as a factor of conversion.
To find the mass in grams of the volume of acetone, multiply the volume by the density (always check the units, that in this case are consistent because 1cm^3=1mL):
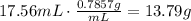
To find the volume of the mass of acetone, divide the mass by the density: