The balanced equation is:
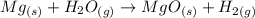
The first step is to convert the given mass of magnesium to moles (MW=24.3g/mol):
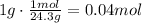
Now, use the ratio given by the chemical equation:
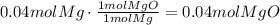
Now, use the molecular weight of magnesium oxide to convert it to grams (MW=40.3g/mol):
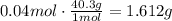
The maximum mass of magnesium oxide produced is 1.612g.