Answer:
0.53L
Explanations:
According to dilution formula:
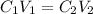
C1 and C2 are the initial and final concentrations
V1 and V2 is initial and final volume
Given the following parameters
• V1 = 45mL
,
• C2 = 0.2M
,
• V2 = 119.4mL
Substitute the given parameters into the formula
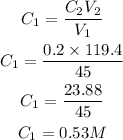
Hence the molarity of the nitric acid is 0.53L