67.57g of calcium metal will be produced.
1st) We need the balanced reaction and the molar mass of calcium hydroxide and calcium metal:
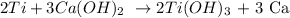
- Ca molar mass: 40 g/mol
- Ca(OH)2 molar mass: 74 g/mol
According to the balanced reaction, we know that 3 moles of calcium hydroxide produces 3 moles of calcium metal.
With the molar mass of the compounds we can convert moles to grams, so we can see that 222g of calcium hydroxide (3 x 74g) can produce 120g of calcium metal (3 x 40g).
2nd) Now we can calculate the amount of calcium metal that will be produced from 125 grams of calcium hydroxide, with a mathematical Rule of Three:
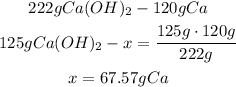
Finally, 67.57g of calcium metal will be produced from 125g of calcium hydroxide.